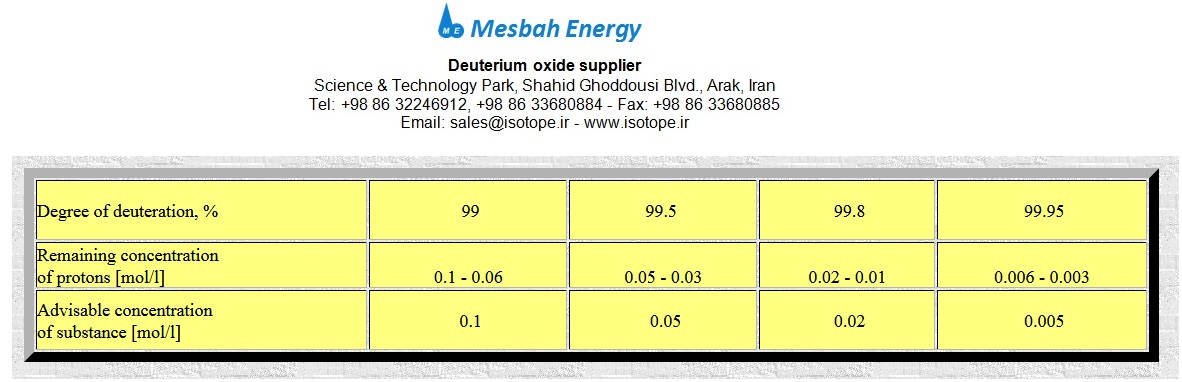
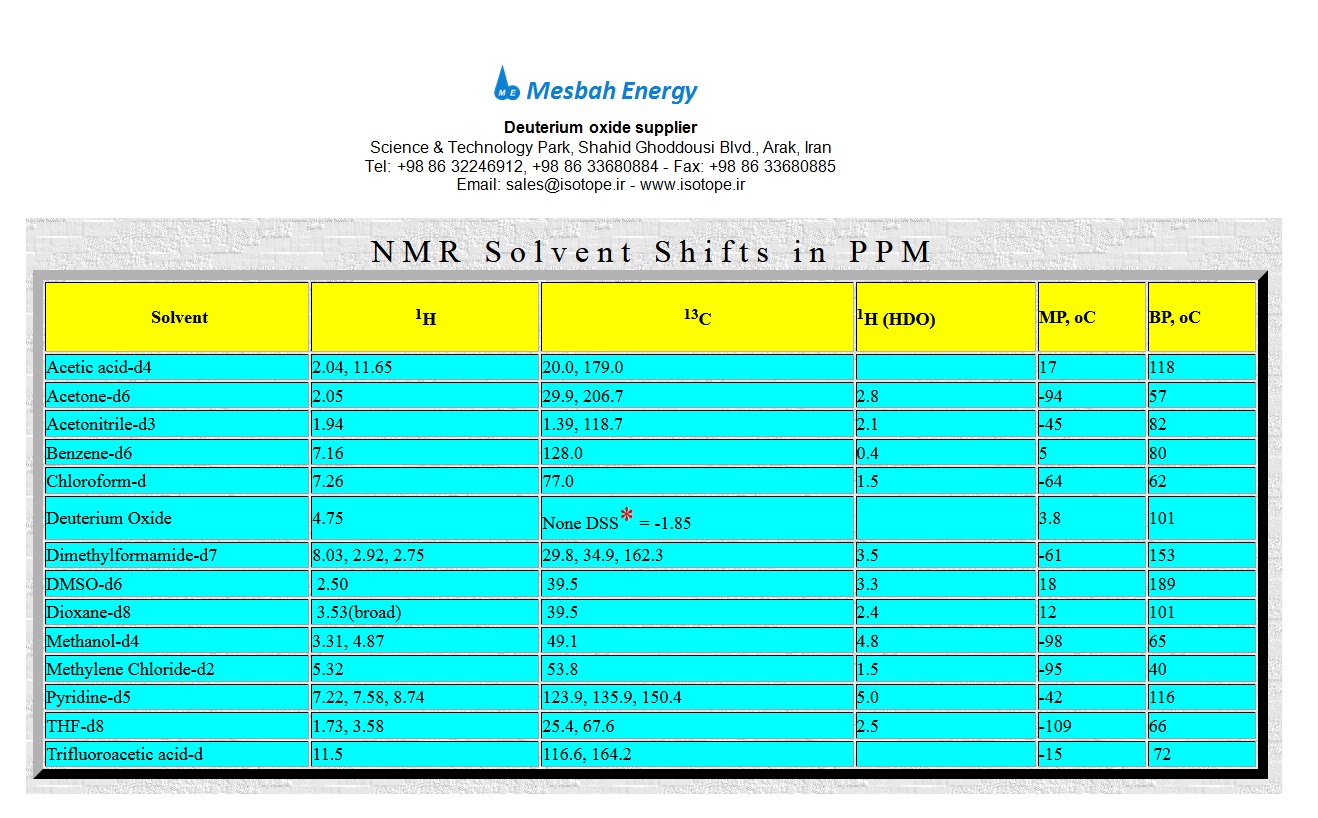
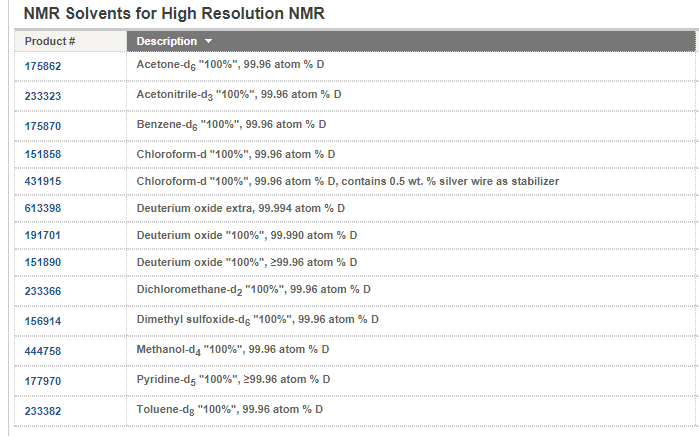
Answer:
Because it is a direct, non-sporting method of chemical analysis, that can directly lead to determination of structure, and of connectivity.
Explanation:
Of course, it has its limitations, but for the vast majority of organic compounds, 1H and 13C{1H} NMR spectroscopies can directly lead to a structural determination given melting points, and molecular weights. Clearly, this task is non-trivial, and regular chemical means of identification (i.e. boiling points, melting points of a few derivatives of known melting points) are required for unequivocal compound identification.
Given that the NMR experiment can be performed on mixtures, the
organic chemist can also use this to assess the course of a reaction.
Has the reaction worked; is the reaction complete or does it need more
time? In the best circumstances, organic mechanisms can be probed in
real time. NMR spectroscopy thus offers a direct means to assess the
extent of chemical reaction.
Ref: https://socratic.org/questions/why-is-nmr-spectroscopy-useful?source=search