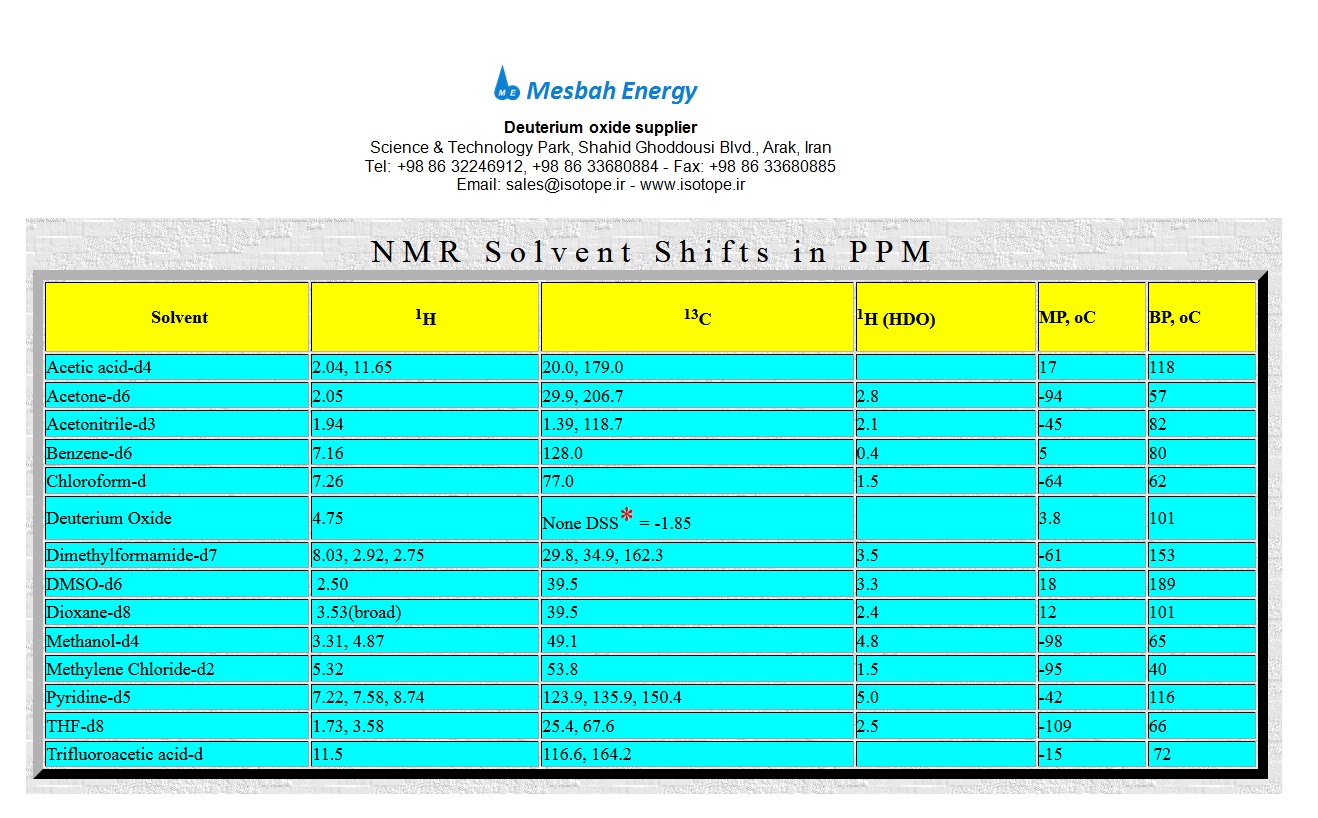
To maintain a strategic distance from spectra ruled by the dissolvable flag, most 1H NMR spectra are recorded in a deuterated dissolvable. Be that as it may, deuteration is never "100%", so motions for the remaining protons are watched. In chloroform dissolvable (CDCl3), this relates to CHCl3, creating a singlet flag is seen at 7.26 ppm. For methanol dissolvable, this compares to CHD2OD, so a 1:2:3:2:1 pentet flag is seen at 3.31 ppm.
Similar solvents are utilized for 13C NMR spectra, so similar standards about part designs apply here moreover. The accompanying table records generally utilized solvents and their substance shifts, which are frequently utilized for recurrence references. The synthetic move information depends on reference to the standard TMS (tetramethylsilane). It is a typical practice to include TMS, or related mixes, as an inner reference standard for 1H and 13C NMR spectra with the proton flag happening at 0.0 ppm and the carbon flag happening at 0.0 ppm in the 13C NMR range.
Basic solvents are accessible in various degrees of deuteration. Signs for water happen at various frequencies in 1H NMR spectra relying upon the dissolvable utilized. Recorded beneath are the concoction move places of the water motion in a few normal solvents. Note that H2O is seen in aprotic solvents, while HOD is seen in protic solvents because of trade with the dissolvable deuterium..
REF :
Physical Data from Handbook of Instrumental Analysis,
NMR Spectroscopy, Merck and Chemical shifts from H-O.Kalinowski,
S. Berger, S. Braun "Carbon-13 NMR Spectroscopy" and
Frank A. Bovey "Nuclear Magnetic Resonance Spectroscopy",
Second Edition