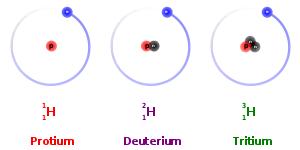
Every chemical element has one or more radioactive isotopes. For example, hydrogen, the lightest element, has three isotopes with mass numbers 1, 2, and 3. Only hydrogen-3 (tritium), however, is a radioactive isotope, the other two being stable. More than 1,000 radioactive isotopes of the various elements are known. Approximately 50 of these are found in nature; the rest are produced artificially as the direct products of nuclear reactions or indirectly as the radioactive descendants of these products.
Radioactive isotopes have many useful applications. In medicine, for example, cobalt-60 is extensively employed as a radiation source to arrest the development of cancer. Other radioactive isotopes are used as tracers for diagnostic purposes as well as in research on metabolic processes. When a radioactive isotope is added in small amounts to comparatively large quantities of the stable element, it behaves exactly the same as the ordinary isotope chemically; it can, however, be traced with a Geiger counter or other detection device. Iodine-131 has proved effective in treating hyperthyroidism. Another medically important radioactive isotope is carbon-14, which is used in a breath test to detect the ulcer-causing bacteria Heliobacter pylori.
Evidence for the existence of isotopes emerged from two independent lines of research, the first being the study of radioactivity. By 1910 it had become clear that certain processes associated with radioactivity, discovered some years before by French physicist Henri Becquerel, could transform one element into another. In particular, ores of the radioactive elements uranium and thorium had been found to contain small quantities of several radioactive substances never before observed. These substances were thought to be elements and accordingly received special names. Uranium ores, for example, yielded ionium, and thorium ores gave mesothorium. Painstaking work completed soon afterward revealed, however, that ionium, once mixed with ordinary thorium, could no longer be retrieved by chemical means alone. Similarly, mesothorium was shown to be chemically indistinguishable from radium. As chemists used the criterion of chemical indistinguishability as part of the definition of an element, they were forced to conclude that ionium and mesothorium were not new elements after all, but rather new forms of old ones. Generalizing from these and other data, English chemist Frederick Soddy in 1910 observed that “elements of different atomic weights [now called atomic masses] may possess identical (chemical) properties” and so belong in the same place in the periodic table. With considerable prescience, he extended the scope of his conclusion to include not only radioactive species but stable elements as well. A few years later, Soddy published a comparison of the atomic masses of the stable element lead as measured in ores rich in uranium and thorium, respectively. He expected a difference because uranium and thorium decay into different isotopes of lead. The lead from the uranium-rich ore had an average atomic mass of 206.08 compared to 207.69 for the lead from the thorium-rich ore, thus verifying Soddy’s conclusion.
The unambiguous confirmation of isotopes in stable elements not associated directly with either uranium or thorium followed a few years later with the development of the mass spectrograph (see mass spectrometry) by Francis William Aston. His work grew out of the study of positive rays (sometimes called canal rays), discovered in 1886 by Eugen Goldstein and soon thereafter recognized as beams of positive ions. As a student in the laboratory of J.J. Thomson, Aston had learned that the gaseous element neon produced two positive rays. The ions in the heavier ray had masses about two units, or 10 percent, greater than the ions in the lighter ray. To prove that the lighter neon had a mass very close to 20 and that the heavier ray was indeed neon and not a spurious signal of some kind, Aston had to construct an instrument that was considerably more precise than any other of the time. By 1919 he had done so and convincingly argued for the existence of neon-20 and neon-22. Information from his and other laboratories accumulated rapidly in the ensuing years, and by 1935 the principal isotopes and their relative proportions were known for all but a handful of
elements.
Ref:
www.britannica.com
NMR solvents are another form (called isotopologue) of organic solvents in which the hydrogen atoms ("H") are replaced with deuterium (heavy hydrogen) isotope ("D"). NMR solvents are common solvent used in NMR spectroscopy.

Isotope Stable:
The atoms of a chemical element can exist in different types. These are called isotopes. They have the same number of protons (and electrons), but different numbers of neutrons. Different isotopes of the same element have different masses. Massis the word for how much substance (or matter) something has. Things with different masses have different weights. Because different isotopes have different numbers of neutrons, they do not all weigh the same or have the same mass.
Different isotopes of the same element have the same atomic number. They have the same number of protons. The atomic number is decided by the number of protons. Isotopes have different mass numbers, though, because they have different numbers of neutrons.
The word isotope, meaning at the same place, comes from the fact that isotopes are at the same place on the periodic table.
In a neutral atom, the number of electrons equals the number of protons. Isotopes of the same element also have the same number of electrons and the electronic structure. Because how an atom acts is decided by its electronic structure, isotopes are almost the same chemically, but different physically to their original atoms.
Heavier isotopes react chemically slower than lighter isotopes of the same element. This "mass effect" is larger for protium (1H) and deuterium (2H), because deuterium has twice the mass of protium. For heavier elements, the relative atomic weight ratio between isotopes is much less, and the mass effect is usually small.
Isotopes are the atoms of the same element that differ in atomic mass, due to difference in the number of neutrons contained in the atom’s nuclei. Isotopes are categorized into two specific types: stable and unstable. For example, the three most abundant isotopes of the Hydrogen are Hydrogen-1 (1H), which contains 1 proton, 1 electron, and 1 neutron; Hydrogen-2 (2H or D), which also has 1 proton and electron, but 2 neutron; and Hydrogen-3 (3H or T) which also contains 1 proton and 1 electron, but 3 neutrons. Having too few or too many neutrons compared to protons causes some isotopes such as 3H to be unstable. These unstable radioisotopes will decay to stable products.
Other isotopes such as 1H and 2H do not decay, because their particular combinations of neutrons are stable. These kinds of isotopes are known as stable isotopes. The relative abundance of stable isotopes in the same compounds from different sources could be different due to the thermodynamic and kinetic effects during chemical and physical process. It can be measured experimentally (isotope analysis), yielding an isotope ratio that is used as a research tool. As a result, stable isotopes have been founding several applications in variety of research areas including the following fields of science:
ref :
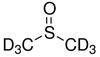
Deuterated DMSO
Deuterated DMSO, also known as dimethyl sulfoxide-d6, is an isotopologue of dimethyl sulfoxide (DMSO, (CH3)2S=O)) with chemical formula ((CD3)2S=O) in which the hydrogen atoms ("H") are replaced with their isotope deuterium ("D"). Deuterated DMSO is a common solvent used in NMR spectroscopy.
Production
Deuterated DMSO is produced by heating DMSO in heavy water (D2O) with a basic catalyst such as calcium oxide. The reaction does not give complete conversion to the d6 product, and the water produced must be removed and replaced with D2O several times to drive the equilibrium to the fully deuterated product.
Use in NMR spectroscopy
Tables of 1 H and 13C NMR chemical shifts have been compiled for common organic compounds often used as reagents or
.found as products or contaminants in deuterated organic solvents
:REF
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a research technique that exploits the magnetic properties of certain atomic nuclei. This type of spectroscopy determines the physical and chemical properties of atoms or the molecules in which they are contained.
Ref : https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance_spectroscopy